Key Clinical Pharmacology Studies to Support Biologic Drug Regulatory Submission
By Sabina Paglialunga, PhD & Aernout van Haarst, PhD; Senior Directors Scientific Affairs, Celerion
Biologic drugs are pharmaceutical products derived from living organisms or their components. They represent a wide range of therapeutic treatments that include monoclonal antibodies, proteins, peptides as well as cell and gene therapies. Oligonucleotide therapeutics, on the other hand, may in principle be synthetic drugs, but by targeting specific RNA sequences to alter RNA and/or protein expression, they share certain features of true biologics. Generally, biologics and oligonucleotide drugs offer several advantages over traditional small molecule products such as targeted therapy, longer half-life (which can be associated with less frequent dosing leading to greater patient adherence) and even higher potency resulting in greater effectiveness. However, unlike most small molecules, plasma pharmacokinetic (PK) profiles of biologics and oligonucleotides may not reflect the target tissue distribution therefore, in some cases appropriate biomarkers or measures of target engagement may need to be assessed as an equivocal dose-effect relationship.
With biologics and oligonucleotide drugs playing an important role in modern medicine, over the past few years the FDA has issued updated guidance for oligonucleotides (draft 2022), peptides (draft 2023) and antibody drug conjugates (ADC; final 2024) to further promote these areas of drug development. Notably, many of the clinical pharmacology studies recommended to support small molecule regulatory submission, as well as efficiencies in corresponding study designs, may also pertain to biologics and oligonucleotide drugs. For example, if safe to do so, enrollment of healthy volunteers (HV) can expedite drug development, as this group is faster to recruit, associated with less variability in PK data, and have no confounding co-morbidities or concomitant medications compared to patients. The below table highlights which key clinical pharmacology studies may be recommended for each drug type.
Table: Key Clinical Pharmacology Studies to Support Drug Regulatory Submission
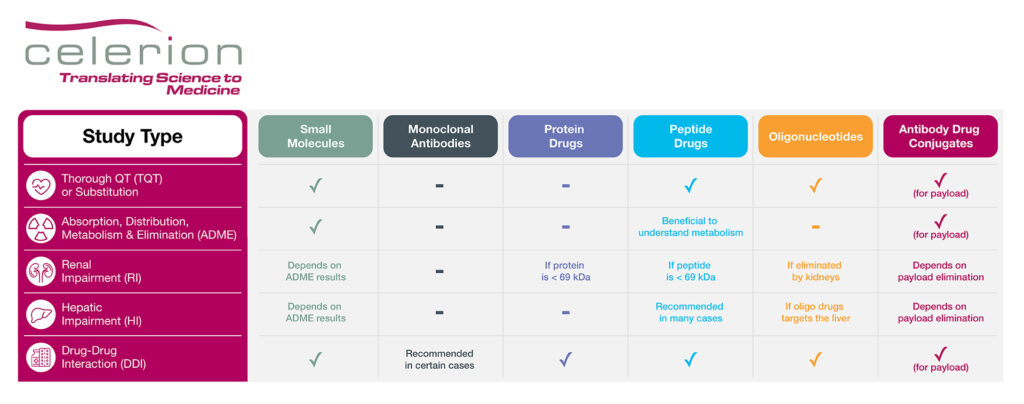
Product Labeling Clinical Pharmacology Studies
While large biologic drugs such as monoclonal antibodies and proteins are exempt from cardiac proarrhythmia risk assessment, a dedicated thorough QT (TQT) study may be recommended for peptide and oligonucleotide drugs, especially if a TQT substitution request via IHC E14 Q&A 5.1 or 6.1 is not sought.
A mass balance study employing a radiolabel is typically recommended for small molecules to understand and track adsorption, distribution, metabolism and elimination (ADME) of the parent drug. While not necessary for biologic and oligonucleotide drugs, there may be cases where an ADME study could be beneficial for peptide products, especially if their distribution and elimination pathways are unknown. The ADME study could also help inform the necessity of organ insufficiency PK studies, such as renal or hepatic impairment studies. For instance, if a protein or peptide drug is < 69 kDa, meaning small enough to be filtered by the kidneys, a renal impairment study is recommended. Similarly, a renal impairment PK study is recommended for oligonucleotide drugs if the therapeutic product is substantially eliminated by the kidneys. In addition, if the oligonucleotide drug targets the liver as part of its mechanism of action, a hepatic impairment PK study should be conducted. Peptides tend to be rapidly degraded by proteases and peptidases, bypassing hepatic elimination, thus negating the need for a hepatic impairment study. However, the recent draft FDA guidance document on peptide drug development does recommend a hepatic impairment PK study under certain conditions, such as:
- The peptide drug is anticipated to undergo substantial hepatic metabolism or biliary excretion
- The (lipid-conjugated) peptide drug is highly bound to serum albumin
- The peptide drug’s pharmacological activity affects normal liver function
Finally, a drug-drug interaction (DDI) study may be recommended if the biologic or oligonucleotide product is a CYP or transporter substrate or modulator, or if a PD interaction with a concomitant medication is anticipated. For example, glucagon like protein-1 (GLP-1) analogs may delay gastric emptying, thereby affecting the absorption of co-administered treatments. In addition, therapeutic proteins that are proinflammatory cytokines or up/down-regulate cytokines levels may also need to be evaluated for DDI potential.
Special Considerations for ADC Drug Development
ADCs combine both small molecule and biologic drug components. The small molecule, often referred to as a ‘payload’, is conjugated to an antibody or an antibody fragment via a chemical linker. The antibody portion of the drug directs the payload to a specific tissue or target cell. Due to the combination of small molecule and biologic drug aspects, clinical pharmacology studies may be recommended to assess the unconjugated payload as well as the ADC or the total antibody, as necessary.
Conclusion
Clinical pharmacology studies such as TQT/cardiac safety, ADME, renal & hepatic impairment or DDI trials may be recommended for biologic and oligonucleotide drug development, depending on the type, size, PK profile and/or PD effects of the product. Celerion’s experienced team of scientific and operational experts are ready to support your biologic drug development program with efficient protocol design, effective study conduct and reliable data management & analysis.
Learn more about our Product Labeling Clinical Pharmacology Studies on our resource page, or contact us at: info@celerion.com.
