How Healthy Volunteer Studies can Accelerate Oncology Drug Development
By Sabina Paglialunga, PhD & Aernout van Haarst, PhD; Senior Directors Scientific Affairs, Celerion
Traditional drugs to treat cancer include chemotherapies, which are cytotoxic agents that non-specifically interrupt cell growth. While chemotherapy drugs effectively obstruct tumor development and growth, they also affect healthy cells, which can result in serious side effects as well as additional malignancies. Over the past two decades, alternative targeted therapies have emerged as less toxic options. Specifically, these drugs are designed to target and inhibit distinct molecular pathways involved in cancer cell growth and survival, such as tyrosine, serine, or threonine kinases. These enzymes and their receptors tend to be overexpressed in tumor cells and are involved in tumor cell proliferation, migration, and angiogenesis.
Compared to conventional chemotherapies, kinase inhibitors are generally considered non-cytotoxic compounds and, therefore, can be administered to healthy volunteers, especially for early-phase clinical pharmacology studies. This approach not only de-risks clinical investigations in patients but can also accelerate drug development. Robust safety and pharmacokinetic (PK) data can be acquired from healthy volunteers for first-in-human (FIH), food effect, and drug-drug interaction (DDI) studies with efficiency, quality, and swiftness.
Advantages of Healthy Volunteers over Patients:
- More resilient human population if adverse events (AEs) occur:
- AEs tend to be transient in nature, and healthy volunteers recover faster than patients do
- Potential for less variable data
- Healthy volunteers are not confounded by comorbidities that could result in data variability
- Healthy volunteers are easier to recruit than most patient populations:
- During early phase development, when efficacy has yet to be established, there is no potential benefit for patients, which may impact motivation to participate in a clinical trial
- Patients are a more fragile population:
- Due to polypharmacy, there may be potential drug interactions
- Effect of disease on AE profiles:
- In patient studies, care must be taken to distinguish drug-related AEs vs natural disease progression
- Time and cost savings:
- Development costs can be substantial as patient studies tend to require multiple sites, and slower recruitment can impact timelines
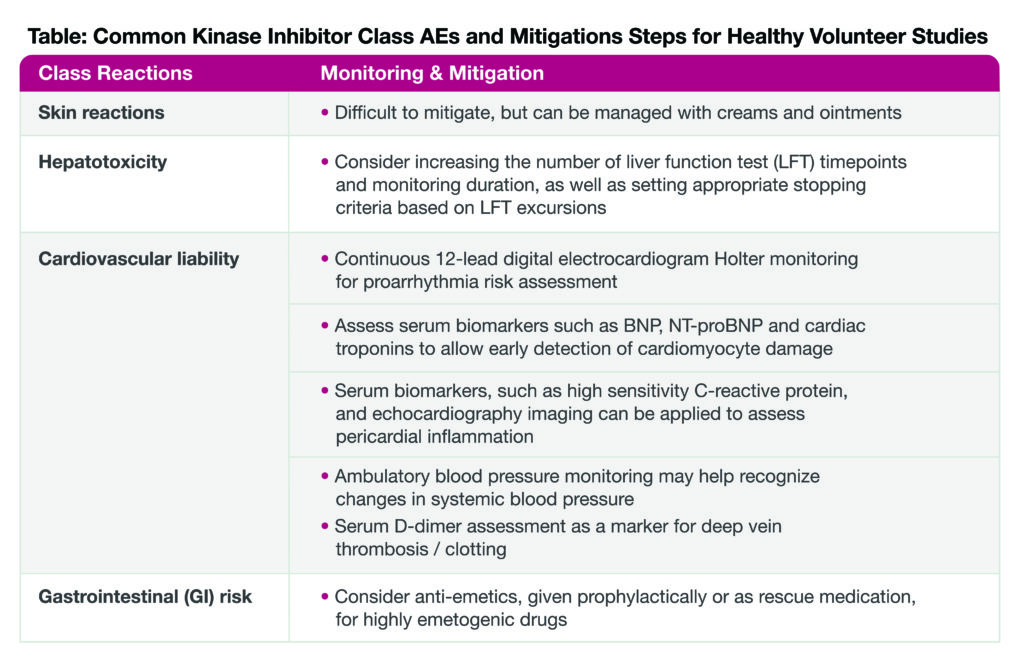
In general, unless the investigational compound causes direct DNA damage, regulatory agencies typically allow the administration of healthy volunteers. Overall, tyrosine kinase inhibitors tend to have an acceptable safety profile that justifies administration in healthy subjects. Nonetheless, they are known to elicit certain adverse effects, such as skin reactions, hepatotoxicity, and cardiovascular and gastrointestinal side effects, yet several mitigation steps can be taken to prevent or overcome these common class AEs.
Product Labeling Studies to Support Regulatory Submission
GI effects are quite common with kinase inhibitors; many cancer patients take acid-reducing agents (ARA) to help manage these side effects. The prevalence of ARA usage ranges between 20-70%, depending upon the cancer type, with GI and pancreatic cancers being the most widespread for ARA treatment. This is relevant for drug developers because of potential drug interactions between the kinase inhibitor and ARAs. In general, ARAs raise the stomach pH, making it a less acidic environment; this could reduce or increase drug bioavailability, which in turn could decrease efficacy or compromise safety, respectively.
Recent FDA guidance provides a physiochemical framework when an ARA-drug interaction study is recommended. Proton-pump inhibitors (PPIs) are the class of ARAs typically evaluated in such DDI studies as they are considered a worst-case scenario due to their relatively strong and long-standing effects on stomach pH. Typically, these PPI studies enroll healthy volunteers and can be combined with a non-medicinal ARA (e.g. orange juice, coke) or food effect arm in a single study design to maximize data output and understand how ‘real word’ factors will impact drug PK.
In addition to ARA DDI trials, other “drug labeling studies” that can be conducted in healthy volunteers include ADME, bioavailability(BA) / bioequivalent (BE), food effect, DDIs, and cardiac safety (TQT) assessments. These studies can support drug label claims and regulatory submissions. Conducting these studies in healthy volunteers allows for robust, high-quality data to be captured quickly.
Conclusions
In general, healthy, normal subjects can be considered for non-cytotoxic, small-molecule clinical trials, such as FIH, ADME, food effect, DDI, and cardiac safety (TQT) studies. Overall, Celerion has conducted over 140 clinical trials with kinase inhibitors since 2010. Relying on our extensive experience, we know how specific risks can best be mitigated. By leveraging data quality and saving time and costs, healthy volunteer oncology studies play a crucial role in accelerating cancer drug development, ensuring that only the most promising candidates proceed to patient populations at the safest dosing regimen.
Learn more about our Product Labeling Clinical Pharmacology Studies on our resource page, or contact us at: info@celerion.com.
