The Rising Tide of Weight Reduction Therapies
Sabina Paglialunga, PhD Senior Director, Scientific Affairs
Weight reduction drugs are making a big splash! Since glucagon-like peptide 1 (GLP-1) receptor agonists were first approved for weight loss in 2021, there has been an estimated 700% increase in prescriptions in the US.1 This has led to a wave of new GLP-1 and incretin products entering into clinical research. Currently, there are nearly 150 novel GLP-1 receptor agonists in various stages of drug development globally.2 Beyond GLP-1, there are several other targets being explored for weight loss, also eager to swim in the same waters. In response, the Food and Drug Administration (FDA) recently updated guidance for industry to support drug development: Obesity and Overweight: Developing Drugs and Biological Products for Weight Reduction
The guidance addresses key aspects for drug developers, including recommendations for early and late phase trials, as well as input on sample size and primary endpoints. The following table highlights important study design elements.
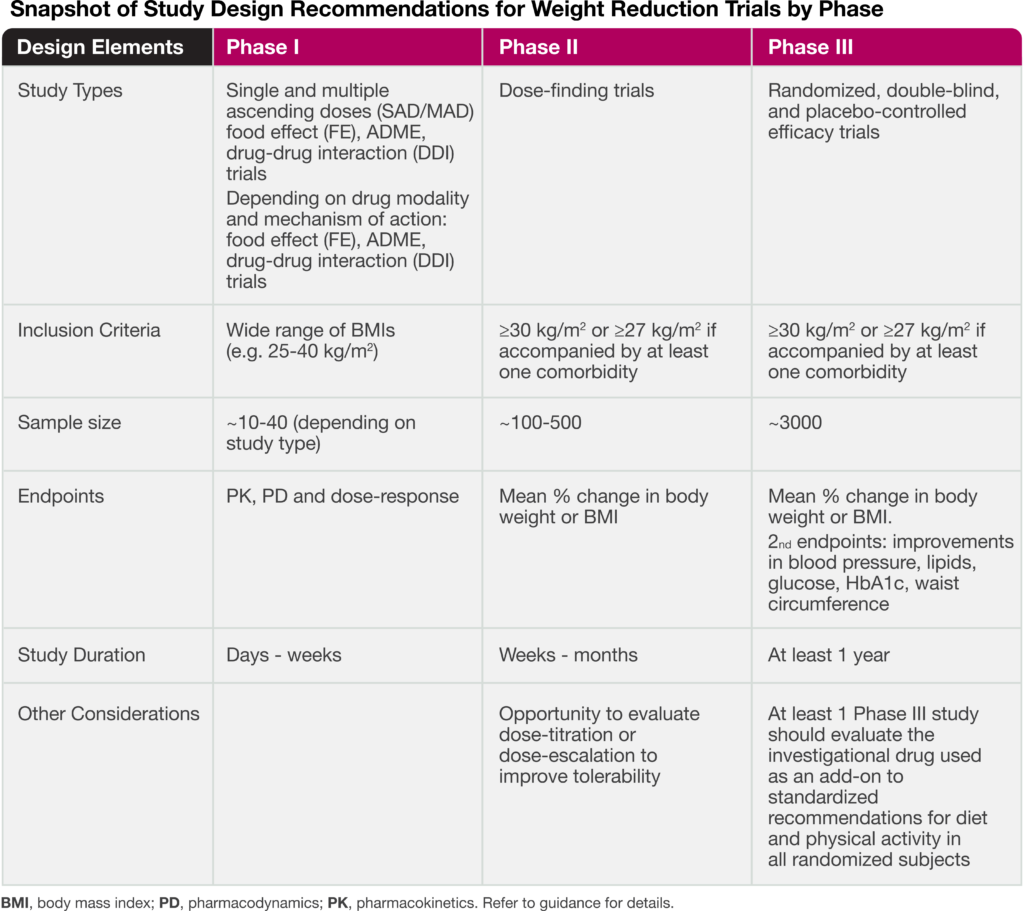
As recently discussed in our blog article: Reframing the Definition of Obesity, the draft guidance also places emphasis on utilizing body mass index (BMI) for inclusion/exclusion criteria as well as the percent change in BMI for a primary endpoint. The FDA recognizes that while BMI is not a direct measure of adiposity (fat mass), it is a simple and effective assessment in which at least a 5% reduction is generally associated with improvement in metabolic and cardiovascular risk factors. In addition, to ensure the weight reduction effect is not due to loss of lean-body mass, body composition assessment by dual x-ray absorptiometry (DEXA) or another imaging modality is highly advised.
Safety Assessments for Weight Reduction Drugs
In terms of safety assessments, the FDA recommends monitoring changes in blood pressure and lipids. Early phase studies, such as SAD/MAD, also provide an opportunity to assess proarrhythmic risk (i.e., QTc prolongation), as well as immunogenicity potential for biologic therapies, including peptide drugs. The guidance also recommends including C-SSRS questionnaires for centrally acting drugs and echocardiographs for serotonin inhibitors.
Combination Products
Common adverse events associated with current GLP-1 therapies include nausea, vomiting, and muscle mass loss. To that end, an emerging trend is to optimize weight loss and minimize potential side effects with combination products. The guidance recommends assessing the safety and PK of each component in Phase I studies prior to initiating late-stage fixed-combination drug products trials.
Diving into Celerion’s Weight Reduction Experience
Celerion has extensive experience with a wide range of compounds for weight reduction, including GLP-1 receptor agonists, insulin sensitizers, and microbiota products. Our comprehensive experience with anti-obesity drugs covers all aspects of development; from first-in-human studies and proof-of-concept trials to clinical pharmacology studies to support labeling, such as drug-drug interaction – and bioavailability/bioequivalence studies.
Conclusion
As research in this indication continues, one can anticipate that the next generation of weight reduction therapies will render better safety profiles, more convenient drug administration (e.g., oral products or less frequent subcutaneous dosing), and improved patient adherence. Celerion is ready to help navigate the regulatory waters and support drug developers by leveraging our early phase clinical research experience, expertise, and efficiencies for smooth sailing ahead!
References
- Gratzl, S et al. Monitoring Report: GLP-1 RA Prescribing Trends – June 2024 Data. medRxiv 2024.01.18.24301500; doi: https://doi.org/10.1101/2024.01.18.24301500
- GlobalData search,14-Feb-2025.
