Rifampin Alternatives for DDI Studies in Response to Nitrosamine Impurities
In August 2020, the FDA reported that marketed batches of rifampin, a semi-synthetic antibiotic used to treat Tuberculosis, were found to contain the nitrosamine MNP above the acceptable intake (AI) limit of 0.16 ppm. From a clinical pharmacology perspective, rifampin had commonly been used in drug development as a CYP3A inducer in drug-drug interaction (DDI) studies. While the FDA temporarily increased the AI limit to 5 ppm for patient use to allow the continuation of receiving this life-saving medication, regulatory agencies including the FDA, EMA and MHRA have prohibited the administration of rifampin with MNP > 0.16 ppm in healthy volunteers. Therefore, alternative CYP3A inducers are needed to continue clinical drug development for DDI studies. In the following articles and webinars, we summarized the root cause of nitrosamines in rifampin as well as the induction potential, study design considerations and safety profile of rifampin alternatives.
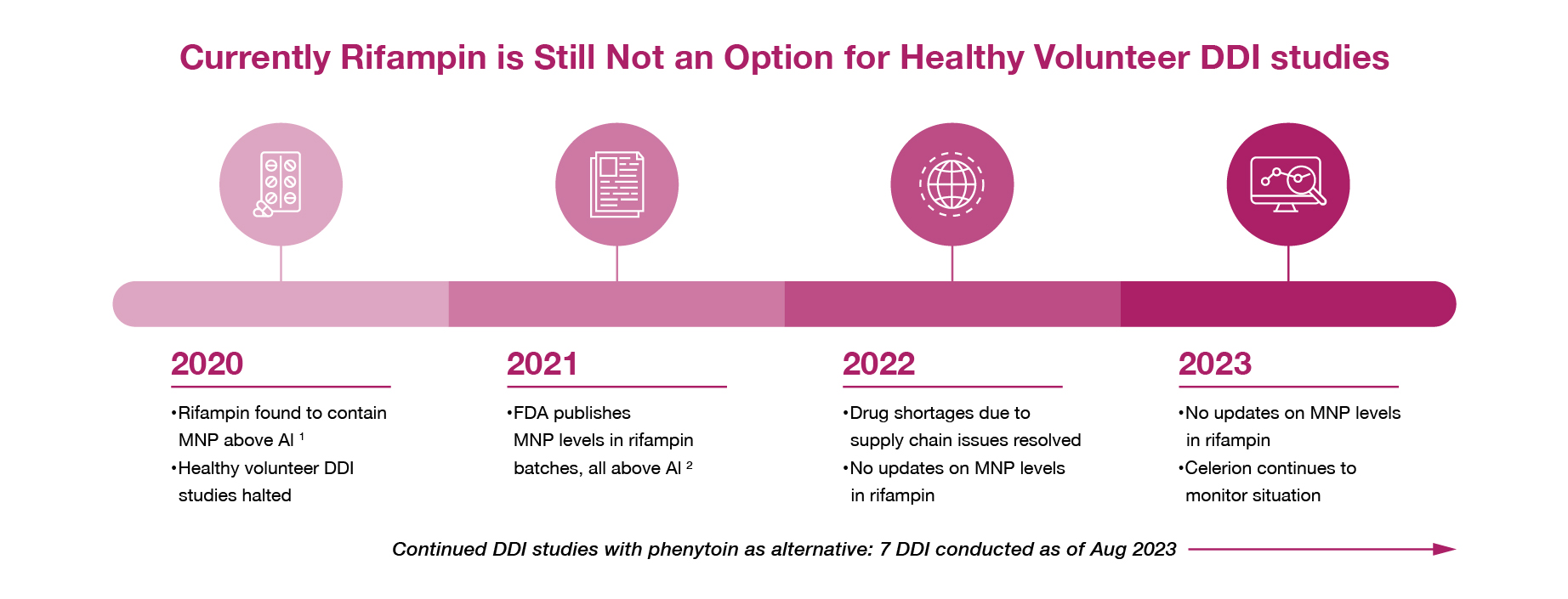
Al, acceptable intake; DDI, drug-drug interaction; FDA, Food and Drug Administration; MP, 1-methyl-4-nitrosopiperazine
