GLP-1 Agonist-Induced Delayed Gastric Emptying – A Clinical Pharmacology Perspective
By Sabina Paglialunga, PhD & Aernout van Haarst, PhD Senior Directors Scientific Affairs
Glucagon-Like Peptide-1 (GLP-1) receptor agonists first came to the market in 2005 as a type 2 diabetes mellitus treatment, and they have been making headlines again for their weight reduction effects. Specifically, semaglutide and tirzepatide, along with diet and exercise, are indicated for weight loss and have been shown to reduce body weight by up to 20%. GLP-1 receptor agonists reduce body weight by decreasing food intake, improving insulin sensitivity as well as delaying gastric emptying. The latter contributes to a longer feeling of fullness.
From a clinical pharmacology perspective, delayed gastric emptying could potentially result in pharmacokinetic (PK) and pharmacodynamic (PD) drug interactions when co-administered with an oral small molecule. Moreover, the importance of evaluating GLP-1 drug interactions due to delayed gastric emptying is highlighted in the FDA’s Drug-Drug Interaction Final Guidance. In addition, we recently addressed the underlying mechanism and potential risks of GLP-1 drug interactions in an ASCPT webinar, highlighting the effect of delayed gastric emptying: That ‘Gut Feeling’: Evaluating the Effects of Food, Gastric pH and Rate of Gastric Emptying on Oral Drug Absorption & Digging into the Impact of Concomitant GLP-1 Agonists.
Assessing Delayed Gastric Emptying
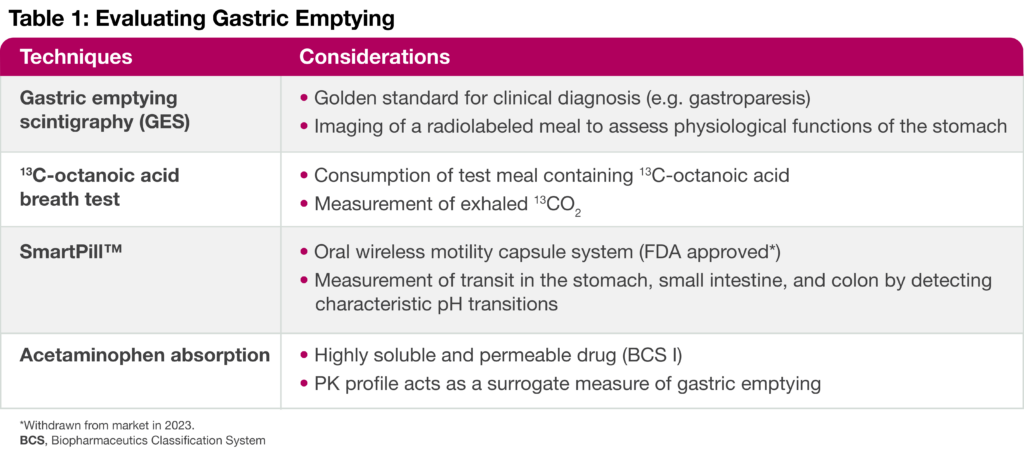
There are several techniques to assess gastric emptying in a clinical setting (Table 1). While scintigraphy is considered the gold standard for diagnostic purposes (e.g. for gastroparesis), an acetaminophen absorption assay applies classic pharmacology study designs to evaluate the role of gastric emptying in a drug-drug interaction study. In addition, the acetaminophen assay could be considered as a better predictor of drug absorption than the other approaches. In this assay, healthy volunteers consume acetaminophen dissolved in yogurt after administration of either a single dose or multiple doses (steady-state) of a drug that putatively alters gastric emptying. Then, changes in the acetaminophen PK profile are compared to a baseline or placebo condition.
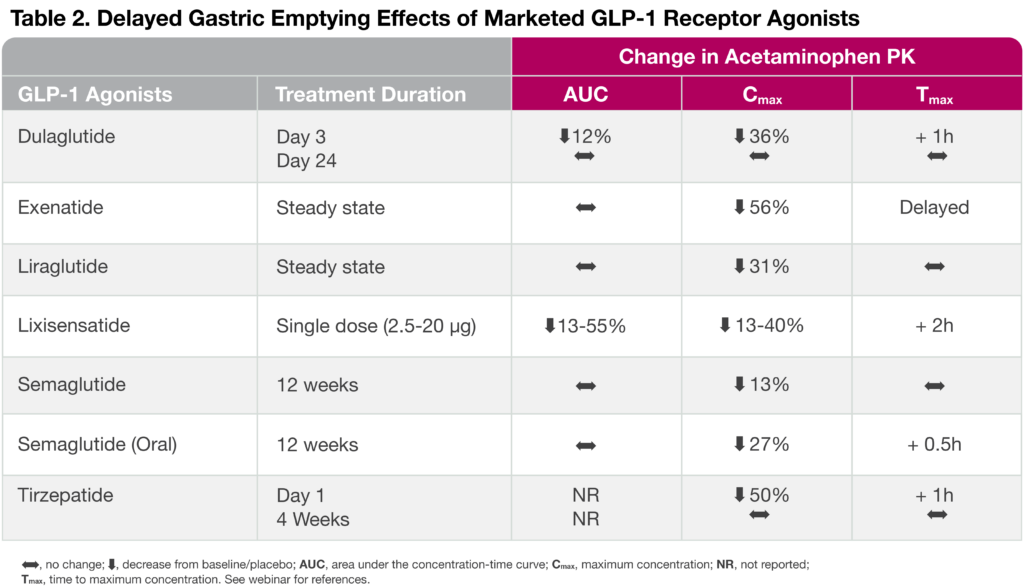
When gauging acetaminophen absorption effects across various marketed GLP-1 receptor agonists, the overall acetaminophen maximum plasma concentration (Cmax) was reduced by 13-56%, a hallmark of delayed gastric emptying (Table 2). Interestingly, the GLP-1 receptor agonist, dulaglutide, and the dual GLP-1 and GIP receptor agonist, tirzepatide, are both characterized by a strong delayed gastric emptying upon a single dose administration, but the effect tends to subside as gastric emptying tachyphylaxis is observed after several weeks of treatment. Nonetheless, the tirzepatide effect of delayed gastric emptying was found to significantly reduce oral contraceptive PK by ~ 60% and, thus, the drug label advises patients using oral hormonal contraceptives to switch to or add a non-oral contraceptive method when initiating tirzepatide and after each dose escalation.
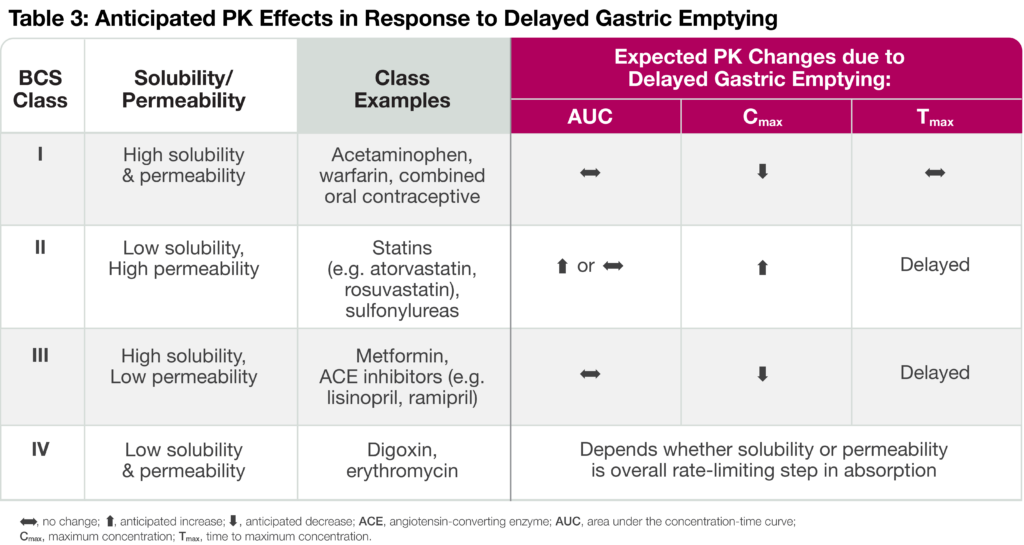
Biopharmaceutics Classification System (BCS) and Delayed Gastric Emptying
The rate of drug absorption depends on the solubility and permeability of an orally administered product. Solubility refers to the dissolution of the drug in an aqueous media, whereas permeability is the ability of a drug to cross membrane barriers and enter into the bloodstream. Oral small molecules, therefore, fall into one of four BCS categories depending on their solubility and permeability characteristics (Table 3). Delayed gastric emptying and reduced gastrointestinal (GI) motility can have different effects on drug PK dependent upon the drug’s BCS category. For instance, Class I drugs like acetaminophen display reduced Cmax in response to delayed gastric emptying. On the other hand, increased residence in the stomach leads to a higher degree of dissolved drug available for absorption immediately after entering the small intestine, resulting in increased PK exposure and delayed time to maximum concentration (Tmax) for Class II drugs. Reduced Cmax and delayed Tmax are anticipated for Class III drugs since intestinal permeability is rate-limiting. Finally, the anticipated PK changes due to delayed gastric emptying depend on whether solubility or permeability is the overall rate-limiting step in absorption.
A clinically relevant effect will depend on the extent of PK changes and the therapeutic index of the co-administered oral drug. For instance, while many of the GLP-1 drug interactions investigated to date may be ‘statistically significant,’ no dose adjustment is required because they were not found to be ‘clinically relevant.’ The one exception is tirzepatide, as described above. Nonetheless, for new GLP-1 drugs in development, it is imperative to evaluate potential drug interactions since this drug class can alter the efficacy of concomitant medications or increase the risk of adverse events as a result of higher exposure to a co-administrated drug.
Conclusion
GLP-1 receptor agonists have been known for their potential to delay gastric emptying. Consequent changes in AUC, Cmax, and Tmax values of orally administered concomitant medications may occur, which could impact drug efficacy or safety. Therefore, if a new oral drug in development is intended to be co-administered with a GLP-1 agonist, we highly recommend conducting a healthy volunteer drug-drug interaction prior to Phase 3 trials to characterize the impact on the PK of the candidate new drug. Similarly, novel GLP-1 receptor agonist-drug interaction studies evaluating the effect of delayed gastric emptying on the PK of oral medications from each BCS class should be considered.
For more information, check out the webinar: Drug Absorption & Impact of Food, Gastric pH, Gastric Emptying and GLP-1 Agonists
