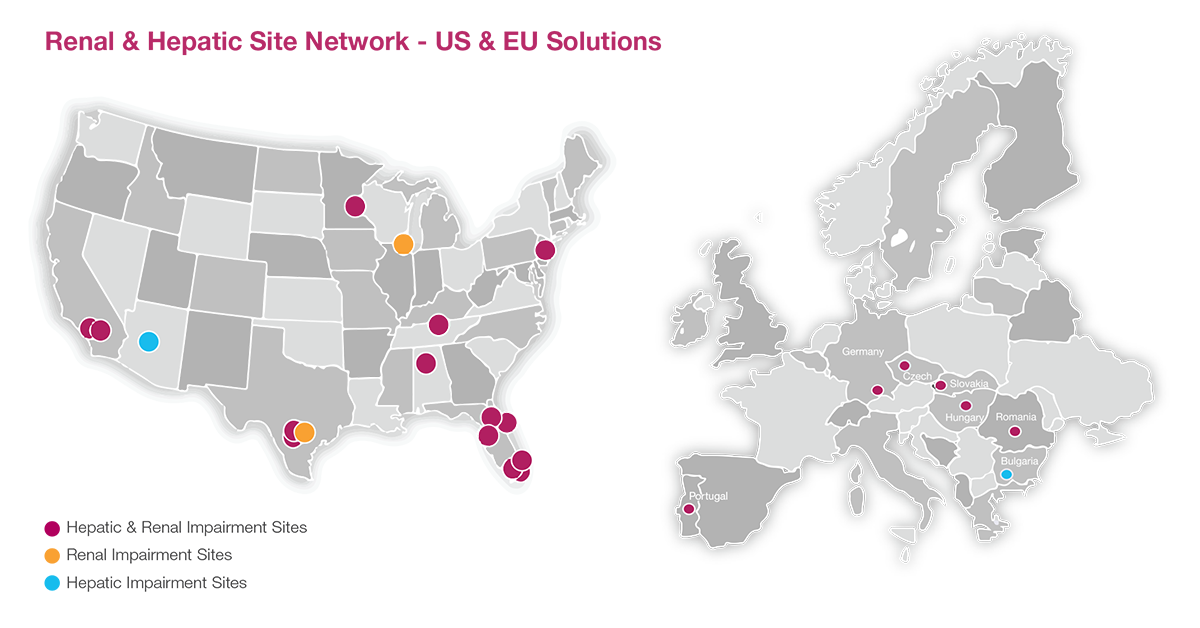
Clinical pharmacology studies, such as ADME, drug-drug interaction (DDI) and renal/hepatic impairment pharmacokinetic (PK) studies can provide important data to support new drug applications (NDAs). While ADME and DDI studies typically enroll healthy volunteer participants, renal/hepatic impairment PK studies require patients, which often adds a degree of complexity to the study design and conduct.
Celerion understands the unique nature of renal and hepatic insufficiency patient populations and manages these specialized PK studies to efficiently and effectively complete your studies on time and with excellent quality data through our extensive US-EU site network. Please see below a wealth of resources that highlight our experience and expertise managing renal/hepatic impairment PK studies.
Renal and Hepatic Services
Discover how Celerion’s expertise ensures success in renal and hepatic insufficiency studies. In this video, Abbey Townsend, Senior Director of Global Clinical Development, explains key challenges and strategies for managing PK studies efficiently and cost-effectively, highlighting our partnerships with top investigators.
Please Complete the Form to Learn More